UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of report (Date of earliest event reported): October 16, 2017
SERES THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 001-37465 | 27-4326290 | ||
| (State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) | ||
| 200 Sidney Street Cambridge, MA |
02139 | |||
| (Address of Principal Executive Offices) | (Zip Code) | |||
Registrant’s Telephone Number, Including Area Code: (617) 945-9626
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01. Regulation FD Disclosure.
On October 16, 2017, Seres Therapeutics, Inc. (the “Company,” “we,” and “our”) posted a slide presentation containing additional statistical analyses and other information related to the Company’s SER-287 program on its website at www.serestherapeutics.com. A copy of the slide presentation is attached as Exhibit 99.1 to this Current Report on Form 8-K and incorporated herein by reference.
The information in Item 7.01 of this Current Report on Form 8-K, including Exhibit 99.1 attached hereto, is intended to be furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such filing. The Company undertakes no obligation to update, supplement or amend the materials attached hereto as Exhibit 99.1.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits.
| Exhibit No. |
Exhibit Description | |
| 99.1 | Seres Therapeutics, Inc. SER-287 Phase 1b Study Results Review Presentation as of October 16, 2017 | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| SERES THERAPEUTICS, INC. | ||||||||
| Date: October 16, 2017 | By: | /s/ Thomas J. DesRosier | ||||||
| Name: Thomas J. DesRosier | ||||||||
| Title: Executive Vice President and Chief Legal Officer | ||||||||

SER-287 Phase 1b Ulcerative Colitis study results review October 2017 Exhibit 99.1

Forward looking statements Some of the statements in this presentation constitute “forward looking statements” under the Private Securities Litigation Reform Act of 1995, including statements on the timing of additional data for the SER-287 Phase 1b study, the efficacy of SER-287, expected interactions with the FDA regarding SER-287, and the application of SER-287 to other diseases. Such statements are subject to important factors, risks and uncertainties (such as those discussed under the caption "Risk Factors" in the Company's Quarterly Report on Form 10-Q filed on August 3, 2017 and its other filings with the SEC) that may cause actual results to differ materially from those expressed or implied by such forward looking statements. Any forward looking statements included herein represent our views as of today only. We may update these statements, but we disclaim any obligation to do so.

PRECLINICAL PHASE 1b PHASE 2 Synthetically fermented Infectious Inflammatory SER-301 Inflammatory Bowel Disease (IBD) SER-262 Primary C. difficile Top line SER-287 Phase 1b data disclosed in October 2, 2017 press release; Collaboration with Nestlé Health Science regarding C. difficile and IBD programs for markets only outside of North America SER-155 Prevention of infection and GVHD following hematopoietic stem cell or solid organ transplant Biologically sourced SER-287 Ulcerative colitis PHASE 3 SER-109 Recurrent C. difficile Research Collaborations CLINICAL PROGRAMS Positive top-line Phase 1b results Robust microbiome therapeutics pipeline

SER-287 overview Biologically sourced consortium of bacterial spores Orally administered Strong intellectual property protection supported by multiple patent claims covering Ulcerative Colitis, as well as regulatory data protection Hypothesized to act by modulating the dysbiotic microbiome, reducing inflammation without immunosuppression effects1,2 Rationale for development supported by: Preclinical results from microbiome modification in animal models of colitis Multiple randomized, placebo-controlled clinical studies examining induction of Clinical Remission of multiple fecal microbiota transplantation in patients with Ulcerative Colitis Blander JM et al., Regulation of inflammation by microbiota interactions with the host, Nature Immunology, 2017. Lynch S and Pedersen O, The Human Intestinal Microbiome in Health and Disease, The New England Journal of Medicine, 2016. Costello et al. Systematic review with meta-analysis: faecal microbiota transplantation for the induction of remission for active ulcerative colitis, Alimentary Pharmacology & Therapeutics, 2017.

Chronic relapsing inflammatory disease affecting colon and rectum Symptoms reflect differences in severity of disease and include: Abdominal pain, bowel urgency, diarrhea, and blood in the stool Ulcerative Colitis Overview Goal of treatment: Induce and maintain long-term remission Mild to moderate: 5-ASAs, immuno-modulators and corticosteroids Moderate to severe: corticosteroids, immuno-modulators, later stage therapies (including anti-TNF antibodies, integrin inhibitors) and surgical resection Large unmet need: Steroids, immuno-suppressants, and biologics have limited efficacy, often with significant safety side effects Unmet need for safe, efficacious, oral therapeutic with a novel non-immunosuppressive mechanistic approach Treatment Approach and Unmet Need Significant Market Potential ~700K patients (U.S.) Kappelman MD et al., The prevalence and geographic distribution of Crohn's disease and ulcerative colitis in the United States, Clin Gastroenterol Hepatol. 2007 Ulcerative Colitis Overview
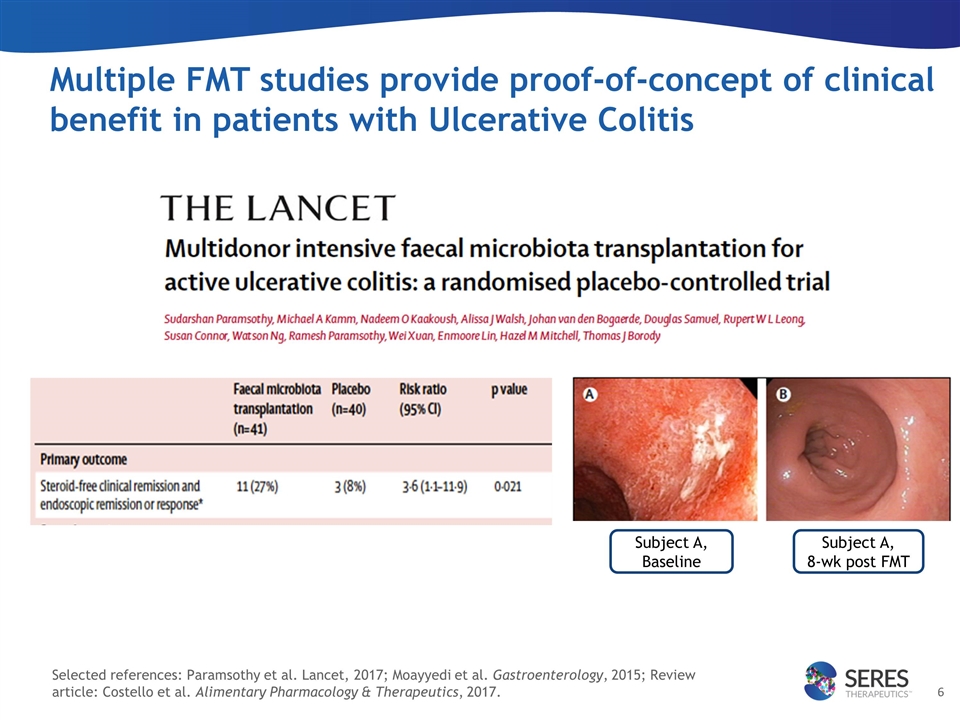
Multiple FMT studies provide proof-of-concept of clinical benefit in patients with Ulcerative Colitis Subject A, Baseline Subject A, 8-wk post FMT Selected references: Paramsothy et al. Lancet, 2017; Moayyedi et al. Gastroenterology, 2015; Review article: Costello et al. Alimentary Pharmacology & Therapeutics, 2017.
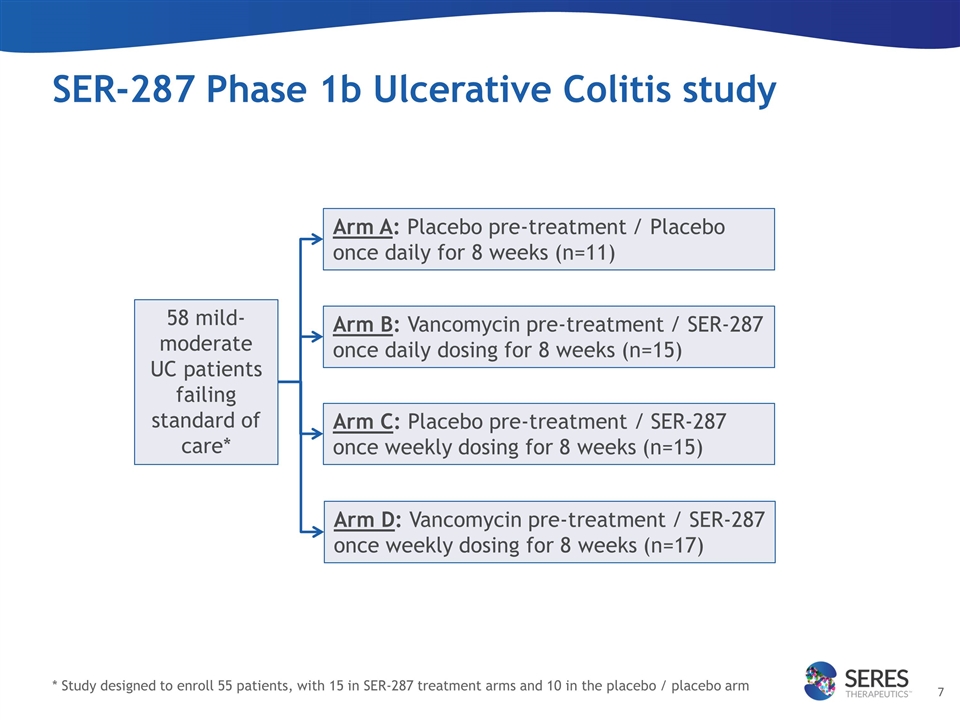
SER-287 Phase 1b Ulcerative Colitis study 58 mild-moderate UC patients failing standard of care* * Study designed to enroll 55 patients, with 15 in SER-287 treatment arms and 10 in the placebo / placebo arm Arm C: Placebo pre-treatment / SER-287 once weekly dosing for 8 weeks (n=15) Arm A: Placebo pre-treatment / Placebo once daily for 8 weeks (n=11) Arm B: Vancomycin pre-treatment / SER-287 once daily dosing for 8 weeks (n=15) Arm D: Vancomycin pre-treatment / SER-287 once weekly dosing for 8 weeks (n=17)

SER-287 Phase 1b study endpoints Primary Objectives Safety and tolerability Change in composition of intestinal microbiome at 8 weeks * Secondary Objectives Clinical remission, endoscopic improvement, and response through measure of the total modified Mayo Score Change in serum and fecal biomarkers * Complement of microbiome metabolic pathways from stool, urine and blood * Immunological and pathologic changes in mucosal biopsies * * Microbiome data and other biomarker data are expected in the coming months

Definitions of clinical efficacy endpoints Endpoint Protocol Definition New FDA Definition (2016)* Clinical Remission Total Modified Mayo Score <=2 and an endoscopic subscore of 0 or 1 Stool Frequency subscore =0, Rectal Bleeding subscore=0 and Endoscopic subscore = 0 or 1(modified) on Mayo Score Endoscopic Improvement Decrease in endoscopic subscore of >=1 Endoscopic subscore = 0 or 1*, but no histological assessment of the mucosa Clinical Response Decrease of >=3 points in Total Modified Mayo Score from baseline, along with either a decrease of >=1 point in rectal bleeding subscore or absolute rectal bleeding subscore of 0 or 1 Not recommended in FDA Guidance *FDA Ulcerative colitis: Clinical Trial Endpoints – Guidance for Industry; August 2016

Pooled estimates of Placebo Clinical Response rates: 33% (95% CI 29-37%) Pooled estimates of Placebo Clinical Remission rates: 10% (95% CI 7-13%) Jairath V. et al., Journal of Crohn's and Colitis, 2016 UC studies have high placebo Clinical Response rates - Not an FDA recommended endpoint

Clinical Remission is now recommended by FDA as the primary endpoint for UC registrational studies “We currently recommend a primary endpoint of clinical remission (responder definition based on Stool Frequency, Rectal Bleeding, and Endoscopy scores).” Clinical Remission is based on objective endoscopy measure, not subjective as would be Clinical Response FDA Ulcerative colitis draft 2016 guidance: Clinical Trial Endpoints – Guidance for Industry Ulcerative Colitis: Clinical Trial Endpoints Guidance for Industry1

Intent-to-Treat statistical analyses Patients analyzed Limited to observed data All patients with post-treatment endoscopy results were included in the efficacy analysis if they remained in the trial until Day 48 Typically used in earlier stage clinical studies 53 / 58 subjects All subjects, missing data counted as failure Patients who discontinued without post-treatment endoscopy or with protocol violations were considered treatment failures in the efficacy analysis Typically used in registrational clinical studies 58 / 58 subjects Two prespecified Intent-to-Treat (ITT) statistical methods were used to analyze SER-287 Phase 1b efficacy outcomes: Initial top-line reported results Note: A patient in the placebo study arm experienced a disease flare and was treated with corticosteroids (a protocol violation) prior to the end of treatment endoscopy. Endoscopy showed improvement and the patient was assessed as having achieved clinical remission per the observed data statistical approach however in the all subjects, missing data approach they were considered a failure due to the protocol violation. SER-287 Phase 1b efficacy analyses
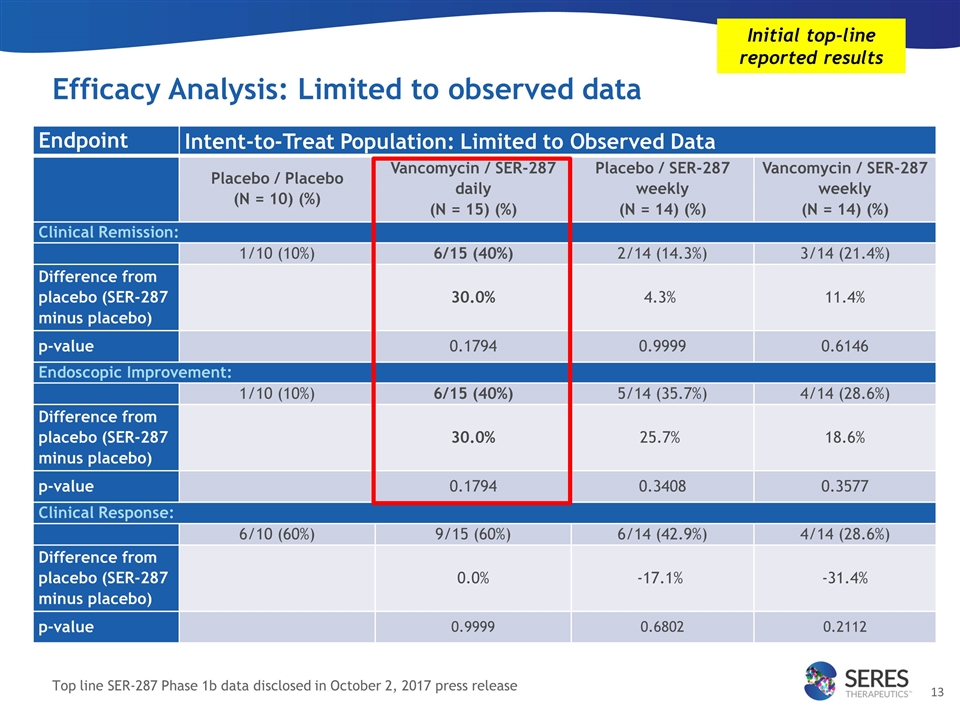
Efficacy Analysis: Limited to observed data Endpoint Intent-to-Treat Population: Limited to Observed Data Placebo / Placebo (N = 10) (%) Vancomycin / SER-287 daily (N = 15) (%) Placebo / SER-287 weekly (N = 14) (%) Vancomycin / SER-287 weekly (N = 14) (%) Clinical Remission: 1/10 (10%) 6/15 (40%) 2/14 (14.3%) 3/14 (21.4%) Difference from placebo (SER-287 minus placebo) 30.0% 4.3% 11.4% p-value 0.1794 0.9999 0.6146 Endoscopic Improvement: 1/10 (10%) 6/15 (40%) 5/14 (35.7%) 4/14 (28.6%) Difference from placebo (SER-287 minus placebo) 30.0% 25.7% 18.6% p-value 0.1794 0.3408 0.3577 Clinical Response: 6/10 (60%) 9/15 (60%) 6/14 (42.9%) 4/14 (28.6%) Difference from placebo (SER-287 minus placebo) 0.0% -17.1% -31.4% p-value 0.9999 0.6802 0.2112 Initial top-line reported results Top line SER-287 Phase 1b data disclosed in October 2, 2017 press release

Efficacy Analysis: All subjects, missing data counted as failure Endpoint Intent-to-Treat Population: All Subjects, Missing Data Counted as Failure Placebo / Placebo (N = 11) (%) Vancomycin / SER-287 daily (N = 15) (%) Placebo / SER-287 weekly (N = 15) (%) Vancomycin / SER-287 weekly (N = 17) (%) Clinical Remission: 0/11 (0%) 6/15 (40%) 2/15 (13.3%) 3/17 (17.7%) Difference from placebo (SER-287 minus placebo) 40.0% 13.3% 17.7% p-value 0.0237 0.4923 0.2579 Endoscopic Improvement: 1/11 (9.1%) 6/15 (40%) 5/15 (33.3%) 4/17 (23.5%) Difference from placebo (SER-287 minus placebo) 30.9% 24.2% 14.4% p-value 0.1783 0.1973 0.6195 Clinical Response: 5/11 (45.5%) 9/15 (60%) 6/15 (40%) 4/17 (23.5%) Difference from placebo (SER-287 minus placebo) 14.5% -5.5% -22.0% p-value 0.6922 0.9999 0.4087

SER-287 safety and tolerability profile very favorable System Organ Class Safety Population (Placebo / placebo) (N = 11) n (%) (Vancomycin / SER-287 daily) (N = 15) n (%) (Placebo / SER-287 weekly) (N = 15) n (%) (Vancomycin / SER-287 weekly) (N = 17) n (%) SER-287 (N = 47) n (%) Gastrointestinal disorders 5 (45.5) 2 (13.3) 7 (46.7) 8 (47.1) 17 (36.2) General disorders and administration site conditions 1 (9.1) 1 (6.7) 0 3 (17.6) 4 (8.5) Immune system disorders 0 0 0 1 (5.9) 1 (2.1) Infections and infestations 3 (27.3) 4 (26.7) 1 (6.7) 6 (35.3) 11 (23.4) Injury, poisoning and procedural complications 2 (18.2) 0 0 0 0 Investigations 0 0 0 1 (5.9) 1 (2.1) Metabolism and nutrition disorders 0 1 (6.7) 0 1 (5.9) 2 (4.3) Musculoskeletal and connective tissue disorders 0 2 (13.3) 3 (20.0) 1 (5.9) 6 (12.8) Nervous system disorders 0 3 (20.0) 0 1 (5.9) 4 (8.5) Psychiatric disorders 1 (9.1) 1 (6.7) 0 0 1 (2.1) Reproductive system and breast disorders 0 0 0 1 (5.9) 1 (2.1) Respiratory, thoracic and mediastinal disorders 0 1 (6.7) 1 (6.7) 2 (11.8) 4 (8.5) Skin and subcutaneous tissue disorders 0 3 (20.0) 0 1 (5.9) 4 (8.5) No drug related serious adverse events associated with SER-287 Lower rate of GI related adverse events in SER-287 daily arm provides supportive evidence of SER-287 benefit on symptoms seen in Ulcerative Colitis

Microbiome results expected in coming months Microbiome analyses to include: Increase in the total bacterial diversity Prevalence of SER-287 spore-former species Identification of bacteria that correlate with efficacy Pending microbiome results to inform: Future SER-287 clinical development plans Additional support for SER-287 mechanism of action Composition of SER-301, a rationally designed, preclinical candidate for Inflammatory Bowel Disease

SER-287 Phase 1b summary: Promising signals of efficacy, with a favorable safety profile SER-287 resulted in a dose-dependent, favorable impact on both Clinical Remission and Endoscopic Improvement Clinical Remission recommended by FDA as the primary endpoint for UC registrational studies Effects on Clinical Response, in this subjective endpoint, were less clear, possibly a result of the modest study size and/or the known high placebo rates observed with Clinical Response Tofacitinib (JAK inhibitor) Phase 2 study for UC, clinical response in the placebo arm was high and several active arms were below, or in the range of the placebo. Separation from placebo for both remission and response was observed in a larger Phase 3 trial. No clinically significant safety or tolerability findings observed

Advancing SER-287 clinical development Company expects to meet with the FDA to determine the most accelerated path to advance SER-287 development in Ulcerative Colitis, both as an induction and a maintenance therapy Company also intends to assess future development in Crohn’s disease and pediatric forms of Inflammatory Bowel Disease (Orphan Indication)

Appendix

Phase 1b study patient demographics Characteristic Statistic (Placebo/ Placebo) (N = 11) (Vancomycin / SER-287 daily) (N = 15) (Placebo/SER-287 weekly) (N = 15) (Vancomycin / SER-287 weekly) (N = 17) Ser-287 (N = 47) Overall (N = 58) Age (years) n 11 15 15 17 47 58 Mean (SD) 45.8 (15.20) 47.8 (18.59) 46.5 (16.12) 47.9 (11.18) 47.4 (15.10) 47.1 (15.00) Sex Male n (%) 4 (36.4) 7 (46.7) 6 (40.0) 10 (58.8) 23 (48.9) 27 (46.6) Female n (%) 7 (63.6) 8 (53.3) 9 (60.0) 7 (41.2) 24 (51.1) 31 (53.4) Race White n (%) 8 (72.7) 12 (80.0) 12 (80.0) 15 (88.2) 39 (83.0) 47 (81.0) Asian n (%) 1 (9.1) 0 0 1 (5.9) 1 (2.1) 2 (3.4) Black or African American n (%) 1 (9.1) 2 (13.3) 3 (20.0) 1 (5.9) 6 (12.8) 7 (12.1) Other - Indian n (%) 1 (9.1) 1 (6.7) 0 0 1 (2.1) 2 (3.4) Severity of UC Mild n (%) 3 (27.3) 6 (40.0) 6 (40.0) 9 (52.9) 21 (44.7) 24 (41.4) Moderate n (%) 8 (72.7) 9 (60.0) 9 (60.0) 7 (41.2) 25 (53.2) 33 (56.9) Receiving Current UC Treatment Prior to or On Date of 1st Pretreatment Dose No n (%) 1 (9.1) 3 (20.0) 2 (13.3) 6 (35.3) 11 (23.4) 12 (20.7) 5-ASA n (%) 7 (63.6) 11 (73.3) 11 (73.3) 9 (52.9) 31 (66.0) 38 (65.5) Immunomodulator n (%) 2 (18.2) 1 (6.7) 4 (26.7) 2 (11.8) 7 (14.9) 9 (15.5) Steroid n (%) 3 (27.3) 3 (20.0) 2 (13.3) 0 5 (10.6) 8 (13.8) Other n (%) 1 (9.1) 0 0 1 (5.9) 1 (2.1) 2 (3.4) Time since first UC diagnosis (months) n 11 15 15 17 47 58 Mean (SD) 138.2 (85.91) 152.9 (143.77) 149.1 (141.34) 142.1 (105.41) 147.8 (127.51) 146.0 (120.12)

SER-287 Phase 1b overall safety: most common (>5%) adverse events by treatment group Safety Population Preferred Term Placebo / Placebo (N = 11) n (%) Vancomycin / SER-287 daily (N = 15) n (%) Placebo / SER-287 weekly (N = 15) n (%) Vancomycin / SER-287 weekly (N = 17) n (%) SER-287 (N = 47) n (%) Treatment-Emergent Adverse Events (All) 7 (63.6) 8 (53.3) 9 (60.0) 14 (82.4) 31 (66.0) Abdominal pain 1 (9.1) 0 3 (20.0) 4 (23.5) 7 (14.9) Nausea 0 1 (6.7) 3 (20.0) 1 (5.9) 5 (10.6) Back pain 0 1 (6.7) 3 (20.0) 1 (5.9) 5 (10.6) Diarrhea 2 (18.2) 0 2 (13.3) 2 (11.8) 4 (8.5) Headache 0 3 (20.0) 0 1 (5.9) 4 (8.5) Constipation 0 2 (13.3) 1 (6.7) 0 3 (6.4) Flatulence 0 0 0 3 (17.6) 3 (6.4) Upper Respiratory Tract Infection 0 2 (13.3) 0 1 (5.9) 3 (6.4)

Mayo Score components Mayo Score Total Modified Mayo Score Stool Frequency 0 = Normal 1 = 1 – 2 stools/day more than normal 2 = 3 – 4 stools/day more than normal 3 = >4 stools/day more than normal Same Rectal Bleeding 0 = None 1 = Visible blood with stool less than half the time 2 = Visible blood with stool half of the time or more 3 = Passing blood alone Same Mucosal Appearance 0 = Normal 1 = Mild disease (erythema, decreased vascular pattern, mild friability) 2 = Moderate disease (marked erythema, absent vascular pattern, friability, erosions) 3 = Severe disease (spontaneous bleeding, ulceration) 0 = Normal 1 = Mild disease (erythema, decreased vascular pattern) 2 = Moderate disease (marked erythema, absent vascular pattern, friability, erosions) 3 = Severe disease (spontaneous bleeding, ulcerations) Physician Rating of Disease Activity 0 = Normal 1 = Mild disease 2 = Moderate disease 3 = Severe disease Same Note: FDA UC guidance (August, 2016) recommends that “The Endoscopy subscore of the Mayo Score should be modified so that a value of 1 does not include friability.”
